The Kase / Tsujimoto Distinguished Professor of Plant & Microbial Biology
We study the photosynthesis of plants, microalgae, cyanobacteria, and photosynthetic bacteria. Approaches include biophysics and biochemistry of the process, molecular biology and genetics of the organisms, and scale ups for product generation. Applied aspects include diverting the flow of photosynthesis to generate high-value compounds instead of the normally produced sugars. Products of interest are biofuels, feedstock for the synthetic chemistry industry and pharmaceuticals. Our trademark is product generation directly from photosynthesis, bypassing the need to harvest and process the respective biomass.
Photosynthetic Bioproducts and Renewable Chemicals
Expertise and Philosophy
The expertise of the Melis lab is in the field of photosynthesis and metabolism. We work with land plants, microalgae, cyanobacteria, and non-oxygenic (anaerobic) photosynthetic bacteria. Our platform includes most aspects of photosynthesis, beginning with organism cultivation, the efficiency of light absorption and utilization, electron transport and biochemical energy generation, and chloroplast and cellular metabolism. Included are the biophysics and biochemistry of the process, the molecular biology and genetics of the organisms, as well as scale ups in the cultivation of the various organisms for product generation.
The concept of “Photosynthetic Bioproducts”, envisioned and pioneered by us, entails the direct application of photosynthesis for the generation of proteins and chemicals, in a process where a single organism acts both as photocatalyst and processor, absorbing and converting sunlight, consuming carbon dioxide, and synthesizing and accumulating ready to use specialty and commodity products.
The lab contributed with a breakthrough in the field, when in 2000 we demonstrated, for the first time, how to divert the natural flow of photosynthesis in green microalgae and to sustainably generate hydrogen gas, instead of the normally produced oxygen. This technology is currently employed by many laboratories in several countries, and serves as the platform for further photobiological hydrogen production research in the field.
The Melis lab also pioneered and currently leads an international effort to improve, by up to 3-fold, the efficiency and productivity of photosynthesis in mass cultures under bright sunlight conditions. This is implemented upon genetically optimizing the size of the array of chlorophyll molecules that serve as antennae to absorb sunlight for the photosynthetic apparatus.
In 2010, the Melis lab pioneered yet a new platform for the renewable generation of isoprene (C5H8) hydrocarbons in cyanobacteria and microalgae, derived entirely from sunlight, carbon dioxide (CO2) and water (H2O), and generated immediately from the primary products of photosynthesis. The process of generating isoprene currently serves as a case study in the development of technologies for the renewable generation of a multitude of natural chemicals, biopharmaceuticals, and other useful bioproducts.
Hydrogen and hydrocarbon fuel and chemicals production via the process of photosynthesis
Current Objectives:
- Maximize the solar-to-biomass energy conversion efficiency of photosynthesis in plants, microalgae, and cyanobacteria cultivated under high mass-culture or canopy-density conditions.
- Apply metabolic engineering approaches to enhance carbon partitioning in photosynthetic organisms toward greater terpenoid biosynthesis.
- Improve the yield of terpene hydrocarbons in plants, microalgae, and cyanobacterial production systems and exploit their photosynthesis for direct "essential oils" production.
- Develop and apply innovative photobioreactor concepts for renewable fuel and chemicals production.
Application of the TLA Concept to Enhance the Solar-to-Biomass Energy Conversion Efficiency of Photosynthesis
The Melis lab has pioneered in plants, microalgae, and cyanobacteria the concept of a Truncated Light-harvesting chlorophyll Antenna size. Such TLA-strains show improved solar-to-biomass energy conversion efficiency of photosynthesis under bright sunlight conditions. The objective of the TLA effort is to approach the theoretical maximum of 8-10% solar-to-biomass energy converion efficiency of photosynthesis, an improvement of up to 300% over the best-case efficiency scenario currrently observed with wild type couterparts.
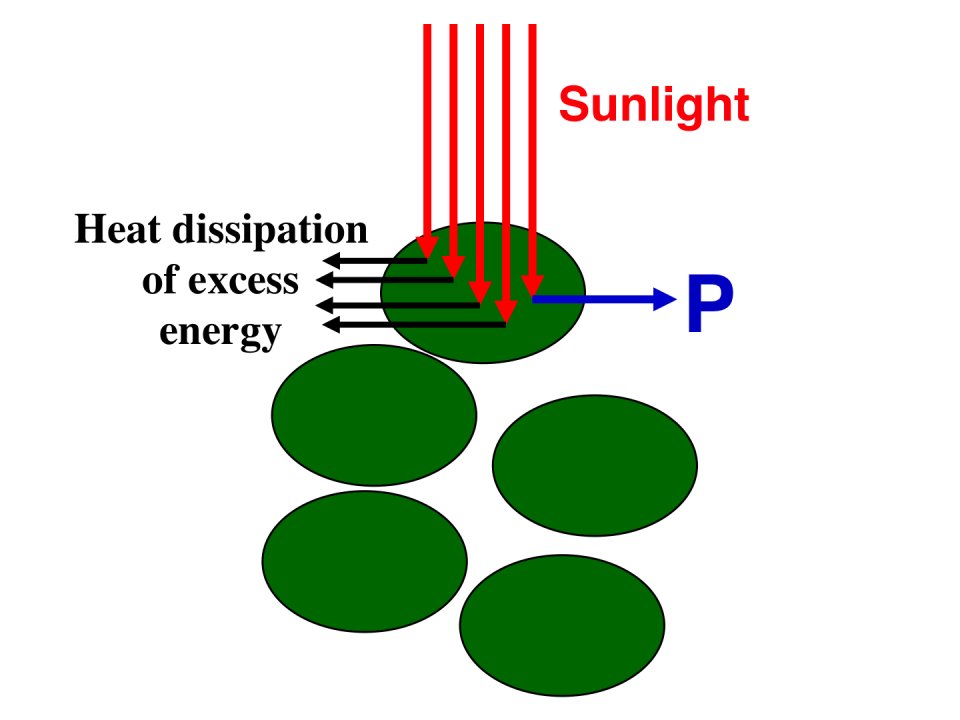
Schematic presentation of incident sunlight absorption and processing by fully pigmented (dark green) microalgae in a high-density culture. Individual cells at the surface of the culture would over-absorb incoming sunlight (more than can be utilized by photosynthesis), and dissipate most of it via non-photochemical quenching (NPQ), thus limiting productivity (P). Note that a high probability of absorption by the first layer of cells would cause shading, i.e., prevent cells deeper in the culture from being exposed to sunlight.
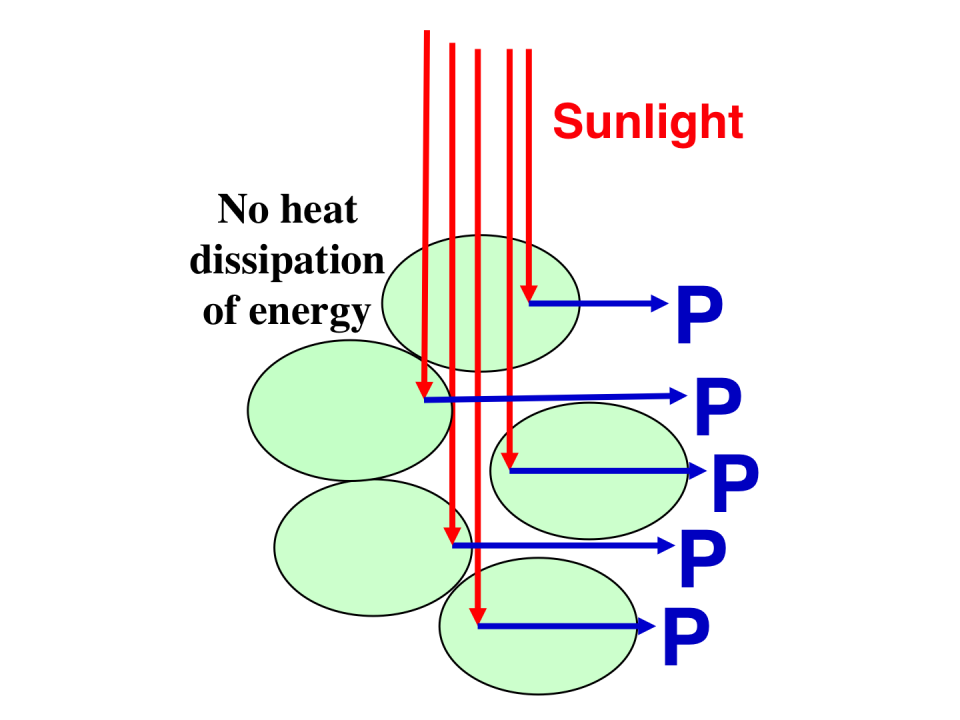
Schematic presentation of incident sunlight absorption and processing through cells with a truncated chlorophyll antenna size. Individual cells have a diminished probability of absorbing sunlight, thereby permitting greater penetration and a more uniform distribution of irradiance through the culture. This alleviates NPQ and enhances photosynthetic productivity (P) by the culture as a whole. (From Melis, 2009.)

A hydrogen-producing Chlamydomonas reinhardtii culture. Hydrogen bubbles emanate toward the surface of the liquid medium. The gas is drained through a syringe (inserted in the middle of the silicone stopper) and, through Teflon tubing, is collected in an inverted burette and measured by the method of water displacement. Photograph courtesy of Michael Barnes, University of California, Office of the President. (From Melis and Happe, 2001.)
Through its Oleomics™ Project, the Melis Lab seeks to identify and exploit genes, enzymens, and biosynthetic pathways leading to "essential oils", including hydrocarbons for fuel and synthetic chemictry feedstock via the photosynthesis of plants, microalgae, and cyanobacteria.
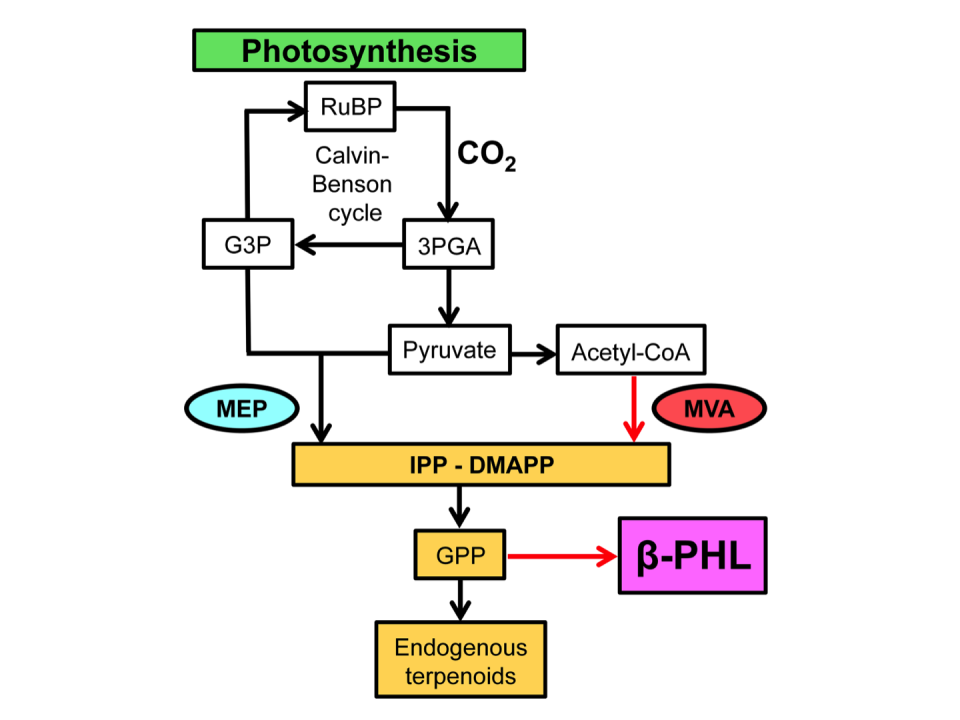
Schematic of metabolic pathways in Synechocystis transformants, as employed in our work. Photosynthetically assimilated CO2 yields 3-phosphoglyceric acid (3PGA), which is converted into glyceraldehyde 3-phosphate (G3P) or pyruvate. Pyruvate and G3P are the primary reactants of the Synechocystis endogenous methylerythritol (MEP) biosynthetic pathway leading to the synthesis of the 5-carbon intermediates isopentenyl-diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Pyruvate decarboxylation leads to acetyl-CoA formation, which is the primary reactant of the heterologous mevalonic acid (MVA) pathway, also leading to the synthesis of IPP and DMAPP. Covalent linkage of IPP and DMAPP yields geranyl-diphosphate, a 10-carbon terpenoid intermediate metabolite, en route toward the generation of longer chain endogenous terpenoids (carotenoids, phytol, and quinone prenyl tails, among other). Heterologous expression of the PHLS gene drains a portion of the GPP pool toward the synthesis of β-phellandrene that spontaneously diffuses out of the cyanobacterial cell. The flow of endogenous carbon substrate toward the terpenoid biosynthetic pathway was enhanced upon heterologous expression of the MVA pathway in Synechocystis, increasing the pool size of IPP and DMAPP substrate. Endogenous and heterologous reactions are delineated in black and red, respectively. (From Formighieri and Melis, 2016.)
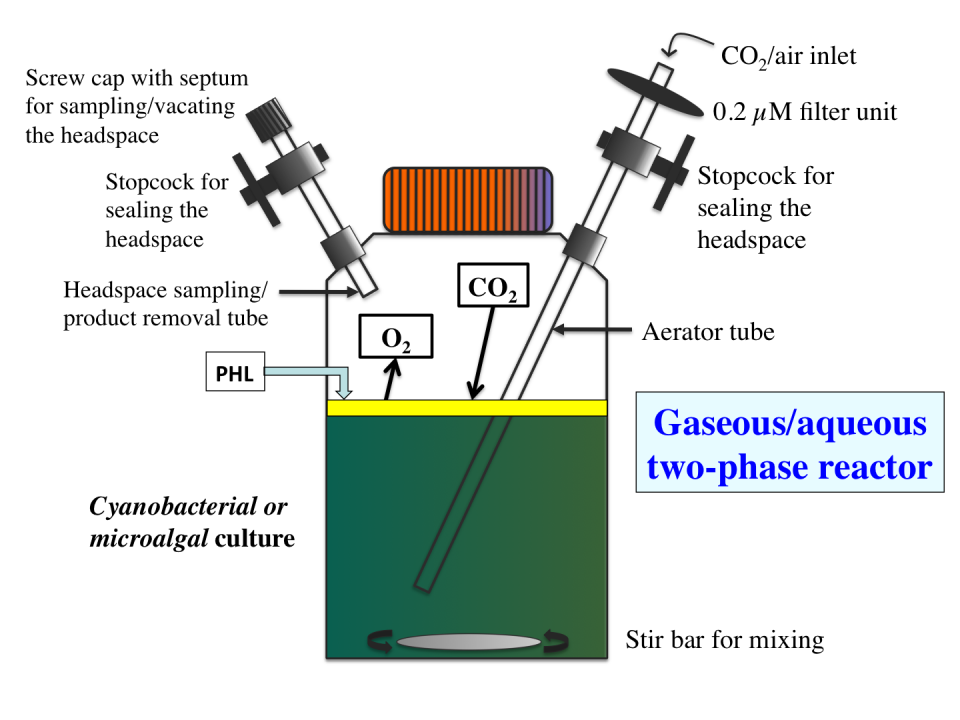
Custom-designed fed-batch bioreactor for diffusion-based gas exchange and terpene hydrocarbons production. A 100% carbon dioxide gas stream was slowly fed into the gaseous/aqueous two-phase bioreactor via the aerator tube to fill the reactor headspace. Efficient and spontaneous uptake and assimilation of headspace carbon dioxide by the cells occurred by diffusion and was concomitantly exchanged for photosynthetically produced oxygen and terpene hydrocarbons during photoautotrophic growth. Beta-phellandrene hydrocarbons accumulate as floater molecules on the surface of the aqueous phase in the sealed bioreactor. (From Bentley and Melis, 2012.)
Formighieri C, Melis A (2015) A phycocyanin•phellandrene synthase fusion enhances recombinant protein expression and β-phellandrene (monoterpene) hydrocarbons production in Synechocystis (cyanobacteria). Metab Eng 32:116–124. http://dx.doi.org/10.1016/j.ymben.2015.09.010
Formighieri C, Melis A (2016) Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth Res 130:123-135. DOI 10.1007/s11120-016-0233-2
Melis A, Bentley FK, Chen Wintz H-C (2016) Diffusion-based method for obtaining volatile hydrocarbons produced by photosynthetic microorganisms in two-phase bioreactors. Australian Patent 2012245238 (issued 10 March 2016; issued 24-April-2018, licensed to the private sector).
Melis A (2016) Maximizing light utilization efficiency and hydrogen production in microalgal cultures. US Department of Energy (DE-FG36-05GO15041) Final Technical Report. DOI: 10.2172/1225978
Eroglu E, Melis A (2016) Microalgal hydrogen production research. Intl. J. Hydrogen Energy 41:12772-12798. doi: 10.1016/j.ijhydene.2016.05.115
Chaves JE, Rueda Romero P, Kirst H, Melis A (2016) Role of isopentenyl-diphosphate isomerase in heterologous cyanobacterial (Synechocystis) isoprene production. Photosynth Res 130:517-527. DOI: 10.1007/s11120-016-0293-3
Baek K, Kim DH, Jeong J, Sim SJ, Melis A, Kim JS, Jin E, Bae S (2016) DNA-free two-gene knockout in Chlamydomonas reinhardtii via CRISPR-Cas9 ribonucleoproteins. SciRep 6:30620. doi: 10.1038/srep30620 (2016).
Jeong J, Baek K, Kirst H, Melis A, Jin E (2017) Loss of CpSRP54 function leads to a truncated light-harvesting antenna size in Chlamydomonas reinhardtii. Biochim Biophys Acta 1858:45–55. doi: 10.1016/j.bbabio.2016.10.007.
Melis A (2017) Terpene hydrocarbons production in cyanobacteria. Chapter 9 in the book entitled Cyanobacteria – Omics and Manipulation. Dmitry A. Los, ed. Caister Academic Press, pp. 187-198. Accession Number: WOS:000388010300010; ISBN:978-1-910190-55-5; 978-1-910190-56-2
Formighieri C, Melis A (2017) Heterologous synthesis of geranyllinalool, a diterpenol plant product, in the cyanobacterium Synechocystis. Appl Microbiol Biotechnol 101:2791-2800 doi: 10.1007/s00253-016-8081-8
Kirst H, Gabilly, ST, Niyogi KK, Lemaux PG, Melis A (2017) Photosynthetic antenna engineering to improve crop yields. Planta 245:1009–1020. 10.1007/s00425-017-2659-y
Chaves JE, Rueda-Romero P, Kirst H, Melis A (2017) Engineering isoprene synthase expression and activity in cyanobacteria. ACS Synth Biol 6:2281-2292 http://dx.doi.org/10.1021/acssynbio.7b00214
Jeong J, Baek K, Jihyeon Yu J, Kirst H, Betterle N, Shin W, Bae S, Melis A, Jin ES (2018) Deletion of the chloroplast LTD protein impedes LHCI import and PSI-LHCI assembly in Chlamydomonas reinhardtii. J Exp Bot. 69:1147-1158. https://doi.org/10.1093/jxb/erx457
Kirst H, Melis A (2018) Improving photosynthetic solar energy conversion efficiency: The truncated light-harvesting antenna (TLA) concept. Chapter 14 in Microalgal Hydrogen Production: Achievements and Perspectives. Seibert M, Torzillo G, Eds. European Society for Photobiology 2018. Published by the Royal Society of Chemistry, London. Pp. 335-353
Betterle N, Melis A (2018) Heterologous leader sequences in fusion constructs enhance expression of geranyl diphosphate synthase and yield of b-phellandrene production in cyanobacteria (Synechocystis). ACS Synth Biol 7:912–921 http://dx.doi.org/10.1021/acssynbio.7b00431
Kirst H, Shen YX, Vamvaka E, Betterle N, Xu DM, Warek U, Strickland JA, Melis A (2018) Downregulation of the CpSRP43 gene expression confers a truncated light-harvesting antenna (TLA) and enhances biomass and leaf-to-stem ratio in Nicotiana tabacum canopies. Planta 248:139–154 DOI 10.1007/s00425-018-2889-7
Chaves JE, Melis A (2018) Engineering isoprene synthesis in cyanobacteria. FEBS Lett 12:2059- 2069; doi:10.1002/1873-3468.13052
Melis A, Bentley F, Wintz Chen H-C, Zurbriggen A (2018) Production of β-phellandrene using genetically engineered cyanobacteria. United States Patent No 9,951,354 (issued 24-April-2018; licensed to the private sector).
Chaves JE, Melis A (2018) Biotechnology of cyanobacterial isoprene production. Appl Microbiol Biotechnol 102(15):6451-6458 https://doi.org/10.1007/s00253-018-9093-3
Formighieri C, Melis A (2018) Cyanobacterial production of plant essential oils. Planta 248(4):933- 946 DOI: 10.1007/s00425-018-2948-0
Chaves JE, Melis A (2018) Synthetic construct beta-phycocyanin linker 7 isoprene synthase fusion (cpcB*L7*IspS) gene, complete cds. GenBank accession number MG855740
Chaves JE, Melis A (2018) Synthetic construct beta-phycocyanin linker 16 isoprene synthase fusion protein (cpcB*L16*IspS) gene, complete cds. GenBank accession number MG855741
Chaves JE, Melis A (2018) Synthetic construct FNI-IPP isomerase protein gene, complete cds. GenBank accession number MG855742
Melis A, Bentley FK, Wintz H-C, Zurbriggen A (2019) Production of β-phellandrene using genetically engineered cyanobacteria. Australian Patent 2013217130 (issued January 24, 2019).
Betterle N, Melis A (2019) Photosynthetic generation of heterologous terpenoids in cyanobacteria. Biotechnology and Bioengineering 116:2041-2051. DOI: 10.1002/bit.26988
Melis A, Kirst H (2019) Decreasing light-harvesting antenna size in cyanobacteria. United States Patent Patent No. 10,385,310 (issued February 11, 2019 and subsequently August 20, 2019).
Melis A, Davies, FK, Chen Wintz, H-C, Zurbriggen A (2020) Production of β-phellandrene using genetically engineered photosynthetic microorganisms. U.S. Patent No. 10,563,228. (issued February 18, 2020).
Betterle N, Hidalgo Martinez DA, Melis A (2020) Cyanobacterial production of biopharmaceutical and biotherapeutic proteins. Front. Plant Sci. 11:237. https://doi.org/10.3389/fpls.2020.00237
Hidalgo Martinez D, Payyavula RS, Kudithipudi C, Shen Y, Xu D, Warek U, Strickland JA, Melis A (2020) Genetic attenuation of alkaloids and nicotine content in tobacco (Nicotiana tabacum). Planta 251:92. https://doi.org/10.1007/s00425-020-03387-1
Valsami E-A, Psychogyiou ME, Pateraki A, Chrysoulaki E, Melis A, Ghanotakis DF (2020) Fusion constructs enhance heterologous β-phellandrene production in Synechocystis sp. PCC 6803. J. Appl. Phycol. 32:2889–2902. https://doi.org/10.1007/s10811-020-02186-1
Formighieri C, Melis A (2020) Fusion constructs as protein overexpression vectors. U.S. Patent No. 10876124 (issued December 29, 2020).
Lu YD, Gan QH, Iwai M, Alboresi A, Burlacot A, Dautermann O, Takahashi H, Crisanto T, Peltier G, Morosinotto T, Melis A, Niyogi K (2021) Role of an ancient light-harvesting protein of PSI in light absorption and photoprotection. Nature Comm 12:679. https://doi.org/10.1038/s41467-021-20967-1
Zhang XN, Betterle N, Hidalgo Martinez D, Melis A (2021) Recombinant protein stability in cyanobacteria. ACS Synth Biol https://dx.doi.org/10.1021/acssynbio.0c00610
Valsami E-A, Pateraki A, Melis A, Ghanotakis DF (2021) Heterologous β-phellandrene production by alginate immobilized Synechocystis sp. PCC 6803. J Appl Phycol in press.
Anastasios Melis
Berkeley, California 94720