What is the role of “jumping genes”?
 By Karyn Houston
By Karyn Houston
Plant & Microbial Biology
Less than 2% of our genome is made up of sequences that produce proteins important for cell function, so what is the role of the remaining 98%?
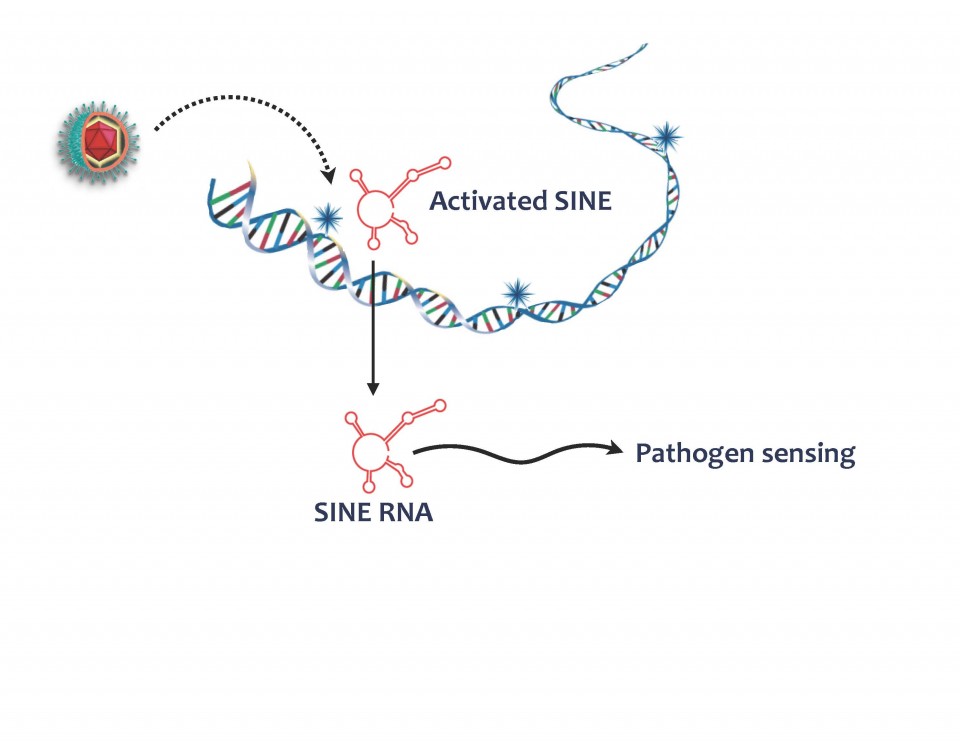
Nearly half of the mammalian genome is composed of transposable elements, also known as “jumping genes” that can copy themselves and move around the genome, in a potentially damaging manner for the cell. For this reason, our cells have found ways to keep these seemingly parasitic sequences turned off—at least most of the time. However, a subset of these elements that do not encode for any proteins, termed short interspersed nuclear repeats, or SINEs, can get activated in response to certain types of stress such as viral infection. When turned on, SINEs produce short segments of ribonucleic acid (RNA). Postdoctoral researcher John Karijolich in the Glaunsinger lab began to wonder whether these SINE RNAs might have been co-opted by cells as an early warning system to alert the cell of a potential incoming virus, because they appear very shortly after incubation of cells with virus.
Postdoctoral researcher John Karijolich in the Glaunsinger lab began to wonder whether these SINE RNAs might have been co-opted by cells as an early warning system to alert the cell of a potential incoming virus, because they appear very shortly after incubation of cells with virus.
Using the mouse herpesvirus MHV68 as a model to study this question, he found that, indeed, SINE RNAs that are generated in response to infection activate part of the cell’s antiviral defenses. In particular, they stimulate a pathway called NF-kB that turns on a multitude of virus-fighting genes in an infected cell.
While the researchers hypothesize that this may help guard the cell against certain types of viruses, it turns out to be a double-edged sword during herpesvirus infection. This is because, surprisingly, the MHV68 herpesvirus is able to subvert this SINE-induced antiviral signal by stealing components of the NF-kB pathway for its own gene expression and replication.
Future studies on how this virus activate SINEs and how these SINE RNAs interface with signaling molecules in the cell may shed light on they myriad of ways our cells attempt to combat viral infection, and how these might be manipulated for therapeutic benefit.
The results of this research are published in the November, 2015, issue of PLOS | Pathogens by authors Karijolich, Emma Abernathy and Glaunsinger.
ABSTRACT:
Short interspersed nuclear elements (SINEs) are highly abundant, RNA polymerase III-transcribed noncoding retrotransposons that are silenced in somatic cells but activated during certain stresses including viral infection. How these induced SINE RNAs impact the host-pathogen interaction is unknown. Here we reveal that during murine gammaherpesvirus 68 (MHV68) infection, rapidly induced SINE RNAs activate the antiviral NF-κB signaling pathway through both mitochondrial antiviral-signaling protein (MAVS)-dependent and independent mechanisms. However, SINE RNA-based signaling is hijacked by the virus to enhance viral gene expression and replication. B2 RNA expression stimulates IKKβ-dependent phosphorylation of the major viral lytic cycle transactivator protein RTA, thereby enhancing its activity and increasing progeny virion production. Collectively, these findings suggest that SINE RNAs participate in the innate pathogen response mechanism, but that herpesviruses have evolved to co-opt retrotransposon activation for viral benefit.
LINKS:
Link to Glaunsinger lab: glaunsingerlab.berkeley.edu
Link to paper: dx.plos.org/10.1371/journal.ppat.1005260
